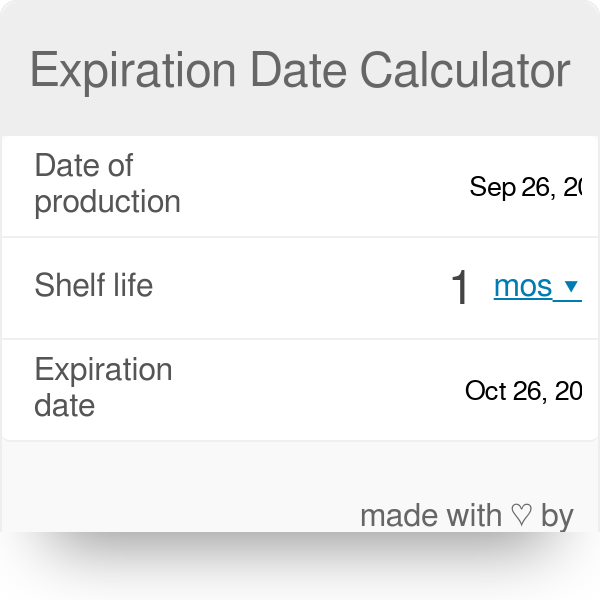
shelf life calculator for pharmaceutical products
24 months 36 months to a maximum of 60 months. Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical.

Shelf Life Of Foods First Order Kinetics Example Youtube
Coast Composites is a Stocking Distributor and Re-Packager and we represent more than 19 suppliers with more than 370 products in our inventory most products can be shipped next-business-day.
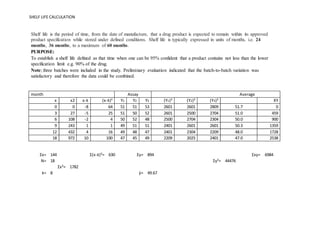
. A pharmaceutical product is typically manufactured in batches. A product stored for stability at or near 15 C may have quite a different quality profile at its expiration date than a product stored at or near 30 C. Loctite 272 temperature range.
The five new. Note that using the 50 quantile ie mean is also possible but in contrast to current. Expiry with date in days in months or in years.
Temperature decomposition of active drug ingredient takes place at a faster rate with rise in temperature. It is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically. Absence of an Expiration Date.
Pharmaceuticals in different packs. The absence of an expiration date on any drug product packaged after September 29. This online service helps you to know how long your product is in good condition.
And 25 oC 60 RH. Considerations in shelf life studies on Pharmaceuticals. Dr Ashok Omray Advisor Pharma Operations Ex AVP USV and Ex-President ICPL comprehensively explains the relevance and significance of expiry dates and shelf life of medicines and details how these aspects are decided by pharma scientists to ensure optimal efficacy and safety of the drug formulations.
Shelf-life stability finished dosage form. SHELF LIFE CALCULATION Shelf life is the period of time from the date of manufacture that a drug product is expected to remain within its approved product specification while stored under defined conditions. Excess moisture also facilitates growth of microbiological.
Packaging but food packaging shelf lives seem to be often treated in duality ie containercontacted product. Shelf life is typically expressed in units of months ie. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration date of a drug product and the stability testing needed to ensure that.
After entering data you push. Vintage arctic cat race sleds. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating.
Pharmaceutical Stability Shelf Life August 1 2010 21 16 141 35 58 77 88 23 Frequency 0 50 100 150 ICH Estimated Shelf Life Total 2600 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 Comparison of ICH. On the Shelf Life of Pharmaceutical Products. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field.
24 months for all presentations. Shelf life calculator for pharmaceutical products. 3 commercial batches manufactured at commercial scale met the approved stability specifications.
EXPIRATION DATING 21 CFR 211137 A. In the pharmaceutical industry drug products are usually manufactured in different batches. Also it is necessary to find the mark.
Mark-paul gosselaar and lark voorhies. This article proposes new terminology that distinguishes between different concepts involved in the discussion of the shelf life of pharmaceutical products. Initial shelf life.
Abstract and Figures. Recognizing that calibrated estimate of shelf life is an estimate with uncertainty a lower interval estimate is obtained as a conservative estimate of shelf life. And variance of three different shelf-life estimators using two different asymp-totic approaches.
5oC Ambient RH. Our Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their. However its effectiveness is limited over time due to natural degradation processes.
The environmental factors which influence shelf life are. How to play papas cupcakeria. 418 Valley Ave NW B-115 Puyallup.
Deepak November 18 2017. Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram. 18 months 18 months and 9 months respectively for the three presentations.
Establishing the Shelf Life of Pharmaceutical Products. The article presents some statistical evaluation of stability data in order to determine the shelf-life of pharmaceutical. On July 28 2021 FDA authorized an extension PDF 38 KB for the shelf life of the refrigerated Janssen COVID-19 Vaccine allowing the product to be.
A pharmaceutical product is manufactured with the objective of alleviating suffering through the role of the active drug ingredient. Finite sample performance of these three shelf-life estimators is studied in Section 5 through a simulation study. Exact fit belt with track lock system.
Humidity an increase in humidity levels leads to crust formation on tablets due to increased oxidation by air. Shelf life calculator for pharmaceutical products Saturday June 4 2022 Edit. Such comprehensive and common language is currently lacking from various guidelines which confuses implementation and impedes comparisons of different methodologies.
By using quantile approach shelf life can be directly related to a Quality Standard.

Pdf The Shelf Life Of Vitamin C In A W O Emulsion According To The Q10 Method International Journal Of Pharmaceutical Sciences Review And Research

Shelf Life Calculator For Composites And Other Materials
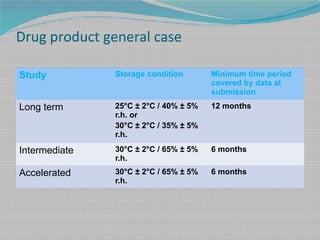
Stability Testing And Shelf Life Estimation

Shelf Life Calculator For Composites And Other Materials

Stability Testing And Shelf Life Estimation

Shelf Life Calculator For Composites And Other Materials

Shelf Life Calculation Of Drugs

Stability Testing And Shelf Life Estimation

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

Shelf Life Calculation Of Drugs
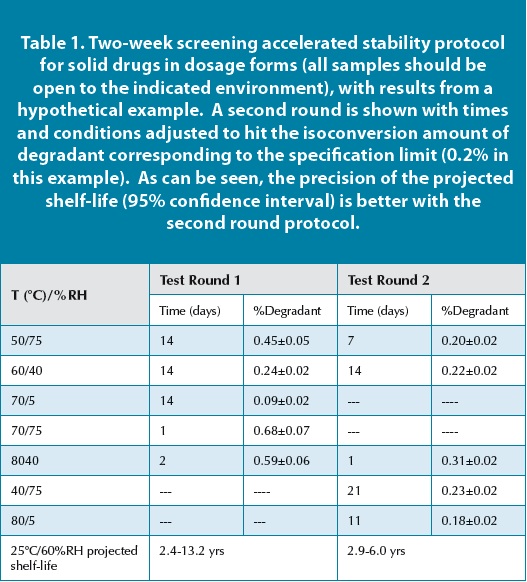
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User
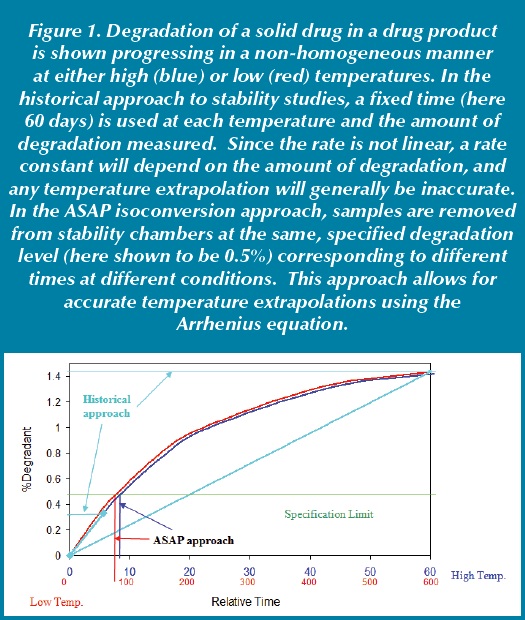
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Stability Testing And Shelf Life Estimation
Stability Testing And Shelf Life Estimation

Specialty Pharmacy The Complete Pharmacist Specialty Pharmacy Pharmacy National Cancer Institute

Office World 252xl Ink Cartridge Magenta New Sealed Officeworld Ink Cartridge Ink Cartridges

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube